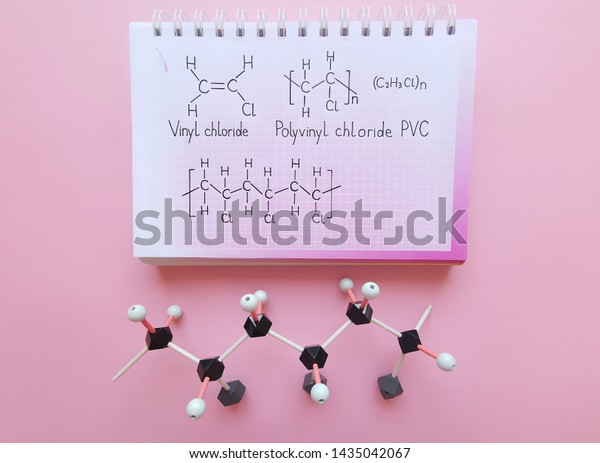
Polyvinyl chloride is produced in an addition polymerisation reaction using the chloroethene vinyl chloride monomer.
Vinyl chloride polyvinyl chloride structure.
It is a thermoplastic having good resistance to alkalis salts and highly polar solvents and it is flame retardant due to the presence of chlorine in the structure.
Vinyl chloride is an organochloride with the formula h 2 c chcl that is also called vinyl chloride monomer vcm or chloroethene this colorless compound is an important industrial chemical chiefly used to produce the polymer polyvinyl chloride pvc.
Pvc comes in two basic forms.
Pvc is used in the manufacture of numerous products including packaging films and water pipes.
Polyvinyl chloride is a white rigid quite brittle solid.
About 13 billion kilograms are produced annually.
However vinyl chloride is mostly unstable thus difficult to store and shows acute toxicity.
Pvc is the world s third most widely produced synthetic plastic polymer after polyethylene and polypropylene about 40 million tons of pvc are produced each year.
Polyvinyl chloride is one of the cheapest and most widely used plastics globally.
Vinyl chloride h2c chcl or c2h3cl n or c2h3cl cid 6338 structure chemical names physical and chemical properties classification patents literature biological activities safety hazards toxicity information supplier lists and more.
Polyvinyl chloride pvc has become a universal polymer with many applications e g for pipes floor coverings cable insulation roofing sheets packaging foils bottles and medical products because of its low cost and physical chemical and weathering properties.
Pvc degrades at relatively low temperatures 100 c in the presence of.
The presence of chlorine makes it flame retardant.
The polymer product of vinyl chloride polyvinyl chloride is stable storable and non toxic.
When treated with certain catalysts vinyl chloride monomers undergo polymerization and form the larger compound known as polyvinyl chloride or pvc.
This compound is important as the monomer for the production of polyvinyl chloride polymer.
Rigid sometimes abbreviated as rpvc and flexible.
Pvc vinyl chloride is an organohalogen compound that has important industrial applications.
Second only to pe in production and consumption pvc is manufactured by bulk solution suspension and emulsion polymerization of vinyl chloride monomer using free radical initiators vinyl chloride ch 2 chcl is most often obtained by reacting ethylene with oxygen and hydrogen chloride over a copper catalyst it is a carcinogenic gas that must be handled with.
Additives are used to modify the properties of polyvinyl chloride to make it more useful.
This polymerisation reaction proceeds by a free radical mechanism.